close
Choose Your Site
Global
Social Media
Views: 24563 Author: Site Editor Publish Time: 2025-09-16 Origin: Site




Polyaspartic polyurea resins represent a class of reactive oligomers derived from the Michael addition of dialkyl aspartates with secondary amines. Distinguished by their low viscosity, adjustable reactivity, and high functionality, these resins have become essential building blocks in modern polyurethane and polyurea technologies. This review summarizes the fundamental chemistry, structural characteristics, physicochemical properties, classifications, and future development trends of polyaspartic polyurea resins.
The demand for high-performance and environmentally compliant resin systems has driven the rapid evolution of polyurea and polyurethane materials. Among them, polyaspartic polyurea resins (also referred to as polyaspartic esters) have attracted considerable attention due to their ability to combine low volatile organic compound (VOC) content with outstanding mechanical and weathering performance. These resins serve as key precursors for advanced polyurea networks, particularly in coatings, adhesives, and elastomeric systems.
The preparation of polyaspartic resins is primarily based on the Michael addition reaction, which involves an electrophilic double bond and a nucleophilic amine. The main precursors include:
Dialkyl maleates or fumarates
Typical examples: diethyl maleate (DEM) and diethyl fumarate (DEF).
These activated esters contain an electron-deficient C=C double bond due to the presence of adjacent ester groups, making them highly susceptible to nucleophilic attack.
Secondary amines
Both aliphatic and aromatic secondary amines can be employed.
Aliphatic amines (e.g., 2-methylpentanediamine, isophorone diamine) provide steric hindrance and moderate reactivity, contributing to longer pot life.
Aromatic secondary amines (e.g., m-phenylenediamine derivatives) accelerate reaction rates but generally yield resins with reduced UV stability.
The central reaction is the conjugate addition of a secondary amine to the activated double bond of the maleate/fumarate ester.
Nucleophilic attack:
The lone pair on the nitrogen atom of the secondary amine attacks the β-carbon of the double bond in the maleate ester.
Intermediate formation:
A carbanion intermediate is transiently formed at the α-carbon adjacent to the ester groups, stabilized by resonance.
Proton transfer:
The carbanion abstracts a proton from the ammonium species, resulting in the formation of a stable β-amino ester structure.
The overall reaction can be summarized as:
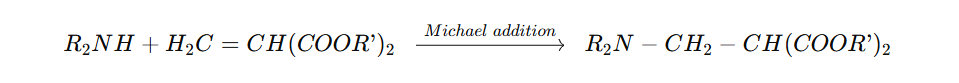
Maleates (cis-isomers) generally react faster than fumarates (trans-isomers), as the cis-orientation facilitates the approach of the amine nucleophile.
The reaction is regioselective, occurring exclusively at the β-carbon of the double bond.
Depending on the secondary amine employed, the resulting polyaspartic ester may contain multiple reactive secondary amine sites available for subsequent reactions with isocyanates.
Temperature: Michael addition typically proceeds at mild conditions (25–60 °C), without the need for strong catalysts. Elevated temperatures accelerate the reaction but may also promote side reactions such as ester hydrolysis.
Catalysts: Although the reaction can occur without catalysts, tertiary amines or organometallic species may be introduced to fine-tune reactivity.
Solvent effects: The synthesis is often carried out solvent-free (bulk polymerization) to reduce VOCs, though polar aprotic solvents (e.g., NMP, DMF) can be employed in laboratory-scale studies.
The resulting polyaspartic ester resin is:
A low- to medium-viscosity liquid (100–1000 mPa·s at 25 °C),
Colorless to pale yellow,
Containing sterically hindered secondary amines.
This steric hindrance is critical: unlike unhindered primary or secondary amines, the nucleophilicity of these hindered amines is significantly reduced, providing controlled reactivity toward isocyanates. This unique property distinguishes polyaspartic resins from traditional amine curing agents.
Conventional secondary amines: exhibit high nucleophilicity, reacting with isocyanates within seconds, often leading to uncontrollably short pot lives.
Polyaspartic resins: steric hindrance lowers the reaction rate, extending the workable time window while still enabling full cure.
This balance between moderated reactivity and high functionality makes polyaspartic resins versatile as reactive building blocks in polyurea and polyurethane networks.
Backbone: derived from aspartate ester moieties.
Reactive sites: sterically hindered secondary amines with moderated reactivity.
Ester functionalities: provide polarity and solubility, improving compatibility with various components.
Appearance: colorless to pale yellow liquid
Viscosity: 100–1000 mPa·s
Amine value: 100–300 mg KOH/g
Density: 1.05–1.15 g/cm³
Storage stability: adequate under dry, dark conditions
Aliphatic-based resins
Derived from aliphatic secondary amines
Offer excellent weather resistance and low yellowing
Suitable for outdoor applications
Aromatic-based resins
Synthesized from aromatic secondary amines
Exhibit higher reactivity and mechanical strength
Limited by inferior UV stability
Modified resins
Incorporation of polyether, polyester, or fluorinated segments
Designed to tailor flexibility, hydrophobicity, or chemical resistance
Polyaspartic resins are of particular scientific importance for several reasons:
They enable the design of low-VOC, high-solid systems.
Their adjustable reactivity allows control over curing kinetics, unlike conventional fast-reacting amines.
They serve as bridging materials, combining the advantages of polyurethanes (versatility, adhesion) with polyureas (durability, rapid cure).
Reactivity control
Precise structural modification to balance pot life and cure speed.
Functional modification
Introduction of hydrophobic, fluorinated, or bio-based segments for enhanced performance.
Sustainability
Development of renewable feedstocks (e.g., bio-based aspartates).
Hybrid systems
Integration with inorganic fillers, nanomaterials, or smart additives for multifunctional properties (self-healing, anti-corrosion, etc.).
Polyaspartic polyurea resins, synthesized via Michael addition of dialkyl aspartates with secondary amines, represent a versatile and sustainable platform for advanced polymer materials. Their low viscosity, tunable reactivity, and superior structural properties continue to make them central to both scientific research and industrial applications. Ongoing studies in structural design, green chemistry, and hybridization are expected to further expand their potential across diverse high-performance material systems.
NEWSLETTER SIGN UP