close
Choose Your Site
Global
Social Media
Views: 12265 Author: Site Editor Publish Time: 2025-10-16 Origin: Site




The contraction force per unit length acting on the surface of a liquid is called surface tension, expressed in units of N·m⁻¹.
The ability of a substance to reduce the surface tension of a solvent is referred to as surface activity, and substances that exhibit this property are called surface-active substances.
Substances that can associate in aqueous solution to form micelles or similar aggregates, possess high surface activity, and exhibit functions such as wetting, emulsifying, foaming, and cleaning are known as surfactants.
Surfactants are a class of organic compounds with unique structures and properties. They can significantly alter the interfacial tension between two phases or the surface tension of a liquid (usually water) and possess functions such as wetting, foaming, emulsifying, and cleaning.
Structurally, all surfactants share a common feature: their molecules contain two parts with different affinities.
One end is a long-chain nonpolar group that is soluble in oil but insoluble in water, known as the hydrophobic group (or lipophilic tail). This hydrophobic part is usually a long-chain hydrocarbon but may also consist of fluorinated, siloxane-based, phosphorous-containing, or organotin chains.
The other end is a water-soluble group, known as the hydrophilic group (or hydrophilic head), which must have sufficient hydrophilicity to ensure that the surfactant as a whole is soluble in water and maintains the required solubility.
Because surfactant molecules contain both hydrophilic and hydrophobic groups, they can dissolve at least in one phase of a liquid system. This dual affinity — being both water-loving and oil-loving — is referred to as amphiphilicity.
Surfactants are amphiphilic molecules that contain both hydrophobic and hydrophilic groups. The hydrophobic group is generally composed of long-chain hydrocarbons, such as linear alkyls (C₈–C₂₀), branched alkyls (C₈–C₂₀), or alkylphenyl groups (with alkyl chains of 8–16 carbon atoms). The differences in hydrophobic groups mainly arise from variations in the hydrocarbon chain structure and are relatively minor, whereas the hydrophilic groups vary greatly in type and structure.
Therefore, the properties of a surfactant depend not only on the size and shape of the hydrophobic group but more importantly on the nature of the hydrophilic group. Since the hydrophilic group exhibits greater structural diversity, the classification of surfactants is generally based on the structure and ionic character of the hydrophilic group.
Accordingly, surfactants are classified into the following main categories:
Anionic surfactants
Cationic surfactants
Nonionic surfactants
Amphoteric (zwitterionic) surfactants
Other special types of surfactants
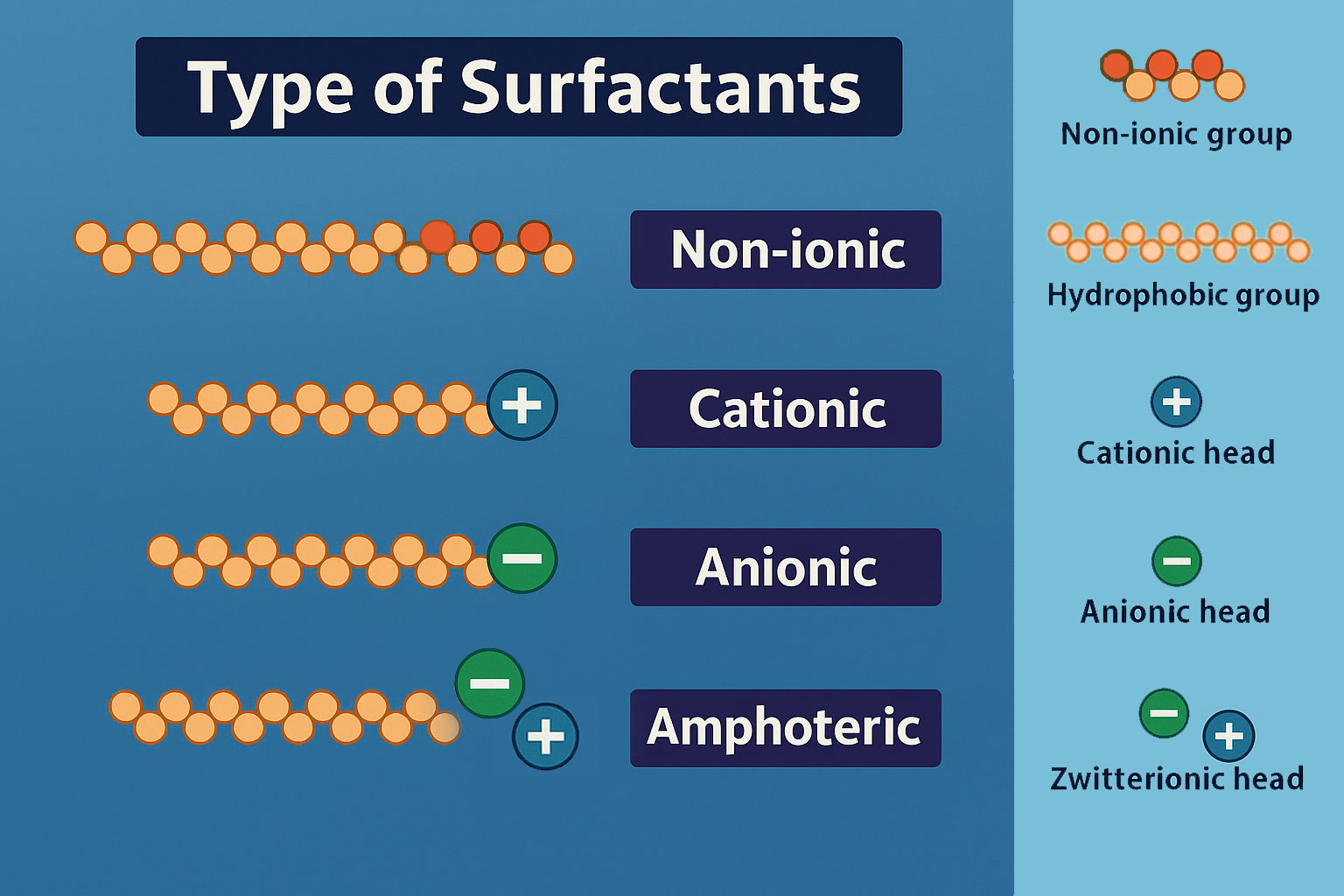
Surfactant molecules are amphiphilic, containing both hydrophilic and lipophilic groups. Water is a highly polar liquid. When a surfactant dissolves in water, based on the principle that like dissolves like — polar groups attract polar substances and repel nonpolar ones — the hydrophilic group interacts with water and dissolves in it, while the hydrophobic group is repelled and moves away from the water phase.
As a result, surfactant molecules (or ions) become adsorbed at the interface between two phases, reducing the interfacial tension. The greater the number of surfactant molecules (or ions) adsorbed at the interface, the greater the reduction in interfacial tension.
Surface Pressure:
When surfactant molecules are adsorbed at the air–liquid interface, they form an adsorbed film. If a frictionless, movable plate is placed on the surface and pushed across the solution, the film exerts a force on the plate — this force is called surface pressure.
Surface Viscosity:
Like surface pressure, surface viscosity is a property exhibited by insoluble molecular films. It can be measured by suspending a fine metal wire holding a platinum ring that touches the surface of a liquid. As the platinum ring oscillates, the damping of the amplitude caused by viscous resistance can be used to determine surface viscosity. First, the test is performed on pure water to measure natural damping; then, the damping is measured again after a surface film is formed. The difference between the two results represents the viscosity of the surface film.
Surface viscosity is closely related to the stability and strength of the surface film. Because the adsorbed film possesses both surface pressure and viscosity, it also exhibits elasticity. The greater the surface pressure and viscosity, the higher the elastic modulus of the film. This elasticity plays an important role in foam stability.
In dilute solutions, surfactants follow the laws of ideal solutions. As the concentration of surfactant increases, the amount adsorbed at the surface also increases. When the concentration reaches or exceeds a certain threshold, adsorption no longer increases. The excess surfactant molecules in the solution do not remain randomly dispersed but rather associate in an orderly manner to form aggregates known as micelles.
Critical Micelle Concentration (CMC):
The lowest concentration of surfactant in solution at which micelles begin to form is called the Critical Micelle Concentration (CMC).
The CMC varies with surfactant type, molecular structure, and solution conditions (temperature, electrolyte content, etc.). In general, ionic surfactants have lower CMC values than nonionic ones, indicating easier micelle formation.
HLB is the abbreviation for Hydrophile–Lipophile Balance, representing the relative balance between the hydrophilic and lipophilic portions of a surfactant molecule. In other words, the HLB value indicates the degree to which a surfactant is hydrophilic or lipophilic.
A high HLB value means the molecule is more hydrophilic and less lipophilic, while a low HLB value indicates stronger lipophilicity and weaker hydrophilicity.
The HLB value is a relative scale. To establish this scale, paraffin (which has no hydrophilic character) is assigned an HLB value of 0, while sodium dodecyl sulfate (which is highly water-soluble) is assigned an HLB value of 40. Therefore, surfactants generally have HLB values in the range of 1–40.
Typically:
Surfactants with HLB < 10 are considered lipophilic and suitable for W/O (water-in-oil) systems.
Surfactants with HLB > 10 are hydrophilic, suitable for O/W (oil-in-water) systems.
The transition point between lipophilic and hydrophilic behavior occurs around HLB = 10.
Based on the HLB value, the potential applications of surfactants can be roughly estimated, as shown below.
Table 1-3 HLB Range and Corresponding Applications
HLB Value Range | Typical Application | HLB Value Range | Typical Application |
1.5–3 | W/O-type defoamer | 8–18 | O/W-type emulsifier |
3.5–6 | W/O-type emulsifier | 13–15 | Detergent |
7–9 | Wetting agent | 15–18 | Solubilizer |
From the table, it can be seen that surfactants suitable for water-in-oil (W/O) emulsifiers have HLB values of 3.5–6, while those for oil-in-water (O/W) emulsifiers have HLB values of 8–18.
(The methods for determining HLB values are omitted here.)
When two immiscible liquids are mixed and one is dispersed in the other in the form of fine droplets or liquid crystals, the resulting system is called an emulsion.
Because the interfacial area between the two liquids increases significantly during emulsification, such a system is thermodynamically unstable. To stabilize it, a third component—an emulsifier—must be added to reduce the interfacial energy.
Emulsifiers are a class of surfactants, and their main function is to promote and stabilize emulsification. In an emulsion, the dispersed phase (or internal/discontinuous phase) is the liquid present as droplets, while the dispersion medium (or external/continuous phase) is the liquid that forms the surrounding medium.
Common emulsions are composed of one aqueous phase (water or aqueous solution) and another phase of organic substances that are immiscible with water, such as oils or waxes.
Depending on the dispersion pattern, emulsions can be classified as:
Oil-in-water (O/W) emulsions: Oil droplets are dispersed in water.
Water-in-oil (W/O) emulsions: Water droplets are dispersed in oil.
Multiple emulsions: More complex systems such as W/O/W (water-in-oil-in-water) or O/W/O (oil-in-water-in-oil) can also be formed.
Emulsifiers stabilize emulsions by reducing interfacial tension and forming a monomolecular interfacial film between the two phases.
Requirements for Effective Emulsifiers
For successful emulsification, an emulsifier must meet the following conditions:
a. Adsorption at the interface: The emulsifier must be able to adsorb or concentrate at the interface between the two phases, thereby reducing interfacial tension.
b. Stabilization of droplets: The emulsifier must impart electrical charges to the droplets to generate electrostatic repulsion between particles, or form a protective film of high viscosity around each droplet to prevent coalescence.
Therefore, only substances that contain both hydrophilic and hydrophobic groups—that is, amphiphilic molecules—can serve as effective emulsifiers. Surfactants satisfy this structural requirement and are the most common and effective emulsifying agents.
There are two main methods for preparing emulsions:
Mechanical dispersion method:
This involves using mechanical force to disperse one liquid into another as fine particles. This is the most commonly used method in industrial applications.
Molecular solution method:
In this approach, one liquid is first dissolved in another at the molecular level and then induced to aggregate under appropriate conditions to form an emulsion.
Stability of Emulsions
The stability of an emulsion refers to its ability to resist droplet aggregation and phase separation.
Since emulsions are thermodynamically unstable systems with relatively high free energy, the so-called “stability”actually describes the time required for the system to reach equilibrium, i.e., the time before phase separation occurs.
When polar organic molecules such as fatty alcohols, fatty acids, or fatty amines are present in the interfacial film, the film strength increases significantly.
This occurs because surfactant molecules interact with these polar compounds to form complexes at the interface, which enhances the interfacial film strength.
Mixed Emulsifiers
When an emulsifier consists of two or more surfactants, it is called a mixed emulsifier.
At the oil–water interface, the different surfactant molecules interact to form complex structures, which greatly reduce interfacial tension, increase surfactant adsorption at the interface, and create a denser, stronger interfacial film, thereby improving stability.
Effect of Droplet Charge
The electric charge of emulsion droplets has a significant influence on stability.
In stable emulsions, droplets typically carry an electric charge. When ionic surfactants are used, their hydrophobic groups embed in the oil phase while their hydrophilic groups remain in the aqueous phase, causing the droplets to become charged.
Since all droplets carry the same charge, they repel each other electrostatically, preventing coalescence and improving stability.
The greater the number of adsorbed surfactant ions on the droplet surface, the higher the charge density and the stronger the resistance to coalescence — resulting in a more stable emulsion system.
Effect of the Viscosity of the Dispersing Medium
The viscosity of the continuous phase also affects emulsion stability.
Generally, a higher viscosity of the dispersion medium leads to greater stability, as it suppresses Brownian motion of droplets and reduces their collisions.
Polymers that dissolve in the emulsion can increase viscosity and thus enhance stability. In addition, polymers can form strong interfacial films, which further stabilize the system.
Effect of Solid Particles
In some cases, adding solid powders can also help stabilize emulsions.
Whether these particles reside in the water phase, oil phase, or at the interface depends on their wettability by oil and water.
If the solid particles are wetted by both water and oil to an appropriate degree, they will accumulate at the oil–water interface, enhancing stability.
The stabilizing mechanism of solid particles is similar to that of surfactant molecules — by forming a compact, solid interfacial layer, they strengthen the interfacial film.
The more densely the particles are packed at the interface, the more stable the emulsion becomes.
Solubilization
After forming micelles in aqueous solution, surfactants exhibit the remarkable ability to increase the solubility of organic substances that are otherwise insoluble or only slightly soluble in water.
At this stage, the solution becomes transparent, and this phenomenon is known as solubilization.
Surfactants capable of producing this effect are called solubilizing agents, while the organic compounds dissolved within micelles are referred to as solubilized substances.
Foam plays an important role in the cleaning and washing process.
Foam is a dispersed system in which gas is dispersed in a liquid or solid medium. The gas is the dispersed phase, while the liquid or solid is the dispersion medium. When the medium is liquid, the system is called a liquid foam; when it is solid, it is called a solid foam, examples of which include foam plastics, foam glass, and foam concrete.
The type of foam discussed here refers to an aggregation of gas bubbles separated by thin liquid films.
Because the dispersed phase (gas) and the dispersion medium (liquid) differ greatly in density, and the liquid typically has low viscosity, gas bubbles quickly rise to the surface of the liquid.
The process of foam formation involves introducing a large amount of gas into a liquid. While most of the bubbles rapidly escape to the surface, a portion of the gas remains temporarily trapped, forming a cluster of bubbles separated by thin liquid films.
Foam exhibits two notable structural characteristics:
Polyhedral bubble shape:
Gas bubbles in foam often take on polyhedral forms. This occurs because at the points where bubbles intersect, the liquid films tend to thin out, forcing the bubbles to become polyhedral. When the liquid film becomes too thin, the bubbles burst.
Need for multiple components:
Pure liquids cannot form stable foam. Foaming requires a system with two or more components.
Aqueous surfactant solutions are typical foaming systems, and their foaming ability is closely related to other properties such as surface tension and viscosity.
Foaming Agents and Foam Stabilizers
Surfactants that have strong foaming ability are called foaming agents.
Although foaming agents can generate foam easily, the resulting foam may not necessarily be stable or long-lasting.
To maintain foam stability, foam stabilizers are often added. These substances help increase the viscosity and strength of the liquid film surrounding the bubbles, thus preventing them from collapsing.
Common foam stabilizers include:
Coconut oil diethanolamide (lauric diethanolamide)
Lauryldimethylamine oxide
Foam is a thermodynamically unstable system. Over time, its natural tendency is for bubbles to collapse, reducing the total surface area of the liquid and thus decreasing the system’s free energy.
The defoaming process involves the thinning and eventual rupture of the liquid film separating gas bubbles. Therefore, the stability of foam mainly depends on the rate of liquid drainage and the strength of the liquid film. Other factors also influence foam stability, as described below.
① Surface Tension
From an energy perspective, low surface tension favors foam formation because it facilitates bubble creation; however, it does not guarantee foam stability.
When surface tension is low, the pressure difference across the film is small, which slows liquid drainage and reduces the thinning rate of the film — thus contributing to foam stability.
② Surface Viscosity
The key factor determining foam stability is film strength, which is primarily governed by the rigidity of the surface adsorption layer, measured by surface viscosity.
Experiments show that solutions with higher surface viscosity produce longer-lasting foam.
This is because stronger intermolecular interactions between surfactant molecules in the adsorption film increase the film’s strength, thereby extending the foam’s lifetime.
③ Solution Viscosity
When the bulk viscosity of the liquid increases, the drainage of liquid from the film becomes more difficult.
As a result, the film thins more slowly, delaying rupture and improving foam stability.
④ “Restorative” Effect of Surface Tension
Surfactant molecules adsorbed on the liquid film surface can resist changes in the film’s surface area — a property known as the restorative effect.
When the film expands, the surface concentration of surfactant molecules decreases, causing an increase in surface tension; further expansion therefore requires more work.
Conversely, when the surface contracts, the surface concentration of surfactant molecules increases, lowering surface tension, which resists further contraction.
This dynamic balance helps maintain the elasticity and integrity of the foam film.
⑤ Gas Diffusion Through the Film
Due to capillary pressure, the internal pressure of small bubbles is higher than that of large bubbles.
This causes gas from smaller bubbles to diffuse through the liquid film into larger ones, leading to the growth of large bubbles and the disappearance of small ones — eventually causing foam collapse.
When surfactants are present, the foam becomes finer and more uniform, and defoaming is suppressed.
This is because surfactant molecules form a dense, compact layer on the film surface, hindering gas diffusion and thereby enhancing foam stability.
⑥ Effect of Surface Charge
If the foam films carry like charges, the two surfaces of the film repel each other, preventing thinning and rupture.
Ionic surfactants can provide this electrostatic stabilization effect.
Summary
In conclusion, film strength is the key factor determining foam stability.
For surfactants acting as foaming agents and foam stabilizers, the compactness and firmness of the adsorbed molecular layer are critical.
When the interactions between adsorbed molecules are strong, the molecular arrangement is tight, resulting in:
A stronger surface film with higher mechanical strength;
Higher surface viscosity, which slows down liquid drainage and maintains film thickness;
Reduced gas permeability, which further enhances foam stability.
Thus, a tightly packed and elastic surfactant adsorption film provides long-lasting, stable foam — an essential property in many cleaning and industrial formulations.
The basic principle of foam destruction is to either alter the conditions under which foam is produced or eliminate the factors that stabilize foam.
Accordingly, there are two main defoaming methods: physical and chemical.
① Physical Defoaming
Physical defoaming involves changing the conditions that favor foam formation without altering the chemical composition of the foaming solution.
Effective physical methods include:
Mechanical disturbance or agitation
Temperature variation
Pressure adjustment
Ultrasonic treatment
These techniques disrupt the foam structure, accelerating bubble coalescence and rupture, thereby eliminating foam.
② Chemical Defoaming
Chemical defoaming involves adding certain substances that interact with the foaming agents, weakening the liquid film and reducing foam stability.
Such substances are known as defoamers (antifoaming agents).
Most defoamers are surfactants that can rapidly spread over the foam surface, reduce surface tension, and destabilize the film.
According to the defoaming mechanism, effective defoamers should have:
A strong ability to lower surface tension,
High surface activity with easy adsorption at the interface, and
Weak intermolecular interactions between adsorbed molecules, leading to a loosely packed molecular layer.
Common Types of Defoamers
Although many substances can function as defoamers, most are nonionic surfactants.
Nonionic surfactants exhibit antifoaming properties near or above their cloud point and are therefore commonly used as defoamers.
Typical defoaming agents include:
Alcohols, especially branched-chain alcohols
Fatty acids and fatty acid esters
Polyamides
Phosphate esters
Silicone oils (silicones)
These compounds effectively reduce surface tension, disrupt film elasticity, and promote rapid bubble collapse, achieving efficient and lasting defoaming performance.
Foam and washing efficiency are not directly related — the amount of foam does not necessarily indicate the effectiveness of cleaning.
For example, nonionic surfactants produce much less foam than soap, yet their detergency (cleaning power) is often superior.
However, under certain conditions, foam can assist in soil removal.
For instance:
During household dishwashing, the foam helps carry away oil droplets detached from the surface.
When cleaning carpets, foam assists in removing dust, powder, and other solid contaminants.
Additionally, foam can sometimes serve as a visual indicator of cleaning progress:
Foam suppression by oily soil: Greasy contaminants inhibit foaming. When there is excessive oil or insufficient detergent, little or no foam forms, or existing foam collapses — signaling that more detergent is needed.
Foam as a rinsing indicator: During rinsing, the amount of foam decreases as residual detergent is removed. Thus, the quantity of foam can be used to estimate how thoroughly the washing or rinsing process has been completed.
In summary, while foam itself does not directly enhance detergency, it can aid cleaning mechanically and serve as a practical indicator of washing and rinsing effectiveness.
In a broad sense, washing refers to the process of removing unwanted components from an object to achieve a specific purpose.
In the usual sense, it means removing dirt or soil from the surface of a substrate (the material being cleaned).
During washing, chemical substances such as detergents act to weaken or eliminate the interactions between the soil and the substrate. This transforms the bond between soil and substrate into a bond between soil and detergent, allowing the soil to be detached from the substrate.
Because both the substrates and types of soils vary widely, washing is a complex physicochemical process.
The basic mechanism of washing can be represented by the following simple relationship:
This shows that the detergent replaces the substrate as the bonding partner of the soil, effectively removing it.
Stages of the Washing Process
The washing process generally occurs in two main stages:
Separation:
Under the action of the detergent, the soil separates from the substrate.
Dispersion and Suspension:
The detached soil is dispersed and suspended in the washing medium(usually water).
However, washing is a reversible process.
The dispersed or suspended soil particles can sometimes re-deposit onto the cleaned surface.
Therefore, an effective detergent must not only have strong ability to remove soil from the substrate, but also possess good dispersion, suspension, and anti-redeposition properties to prevent the soil from returning to the cleaned surface.
Even for the same type of object, the kind, composition, and amount of soil may vary greatly depending on the usage environment.
Oily soils mainly include animal and vegetable oils and mineral oils (such as crude oil, fuel oil, and coal tar).
Solid soils consist primarily of soot, dust, rust, and carbon black.
For textiles or clothing, soils can originate from many different sources:
Human body: sweat, sebum, and blood.
Food: fruit stains, cooking oil, sauces, and starch residues.
Cosmetics: lipstick, nail polish, etc.
Atmospheric sources: smoke, dust, and mud.
Others: ink, tea, paint, and more.
In short, soils are diverse in type and composition.
Generally, soils can be divided into three major categories: solid soils, liquid soils, and special soils.
① Solid Soils
Common solid soils include dust, mud, clay, rust, and carbon black particles.
Most of these particles carry electrical charges, usually negative, which makes them easily adsorbed onto fibers or surfaces.
Solid soils are typically insoluble in water, but can be dispersed and suspended by detergent solutions.
The smaller the soil particles, the more difficult they are to remove.
② Liquid Soils
Liquid soils are generally oil-soluble, including animal and vegetable oils, fatty acids, fatty alcohols, mineral oils, and their oxidized derivatives.
Animal and vegetable oils, as well as fatty acids, can undergo saponification reactions with alkalis.
Fatty alcohols and mineral oils, however, do not saponify, but can dissolve in organic solvents such as alcohols, ethers, and hydrocarbons, and can be emulsified and dispersed by aqueous detergent solutions.
Oil-soluble liquid soils tend to have strong adhesion to fibers and are therefore more difficult to remove.
③ Special Soils
Special soils include proteins, starch, blood, human secretions (such as sweat, sebum, and urine), as well as fruit juice, tea stains, etc.
These soils often bind tightly to fibers through chemical interactions, making them more challenging to clean.
In reality, different soils rarely exist in isolation — they are often mixed together and adsorbed simultaneously on the surface of an object.
Moreover, under environmental influences, soils can undergo oxidation, decomposition, or decay, leading to the formation of new contaminants that are even harder to remove.
Clothing, skin, and other surfaces become soiled because there are interactions between the soil and the substrate.
The adhesion of soils can be broadly divided into physical adhesion and chemical adhesion.
① Physical Adhesion
Physical adhesion occurs when particles such as soot, dust, mud, and carbon black attach to fabrics or surfaces.
In general, this type of adhesion involves relatively weak forces, making it easier to remove compared with chemical adhesion.
Depending on the nature of the forces involved, physical adhesion can be further classified into mechanical adhesion and electrostatic adhesion.
A. Mechanical Adhesion
This type mainly refers to the attachment of solid particles (such as dust and sand) to a surface.
It is the weakest form of adhesion, and such soils can often be removed by simple mechanical means (e.g., shaking or brushing).
However, when the soil particles are very small (less than 0.1 μm), they are much harder to remove due to their high surface area and stronger physical attraction.
B. Electrostatic Adhesion
Electrostatic adhesion occurs when charged soil particles are attracted to surfaces of opposite charge.
Most fibrous materials become negatively charged in water and thus easily attract positively charged particles, such as lime (CaO, CaCO₃).
Some negatively charged soils, like carbon black particles in aqueous solution, can still adhere to fibers through ionic bridges — where positive ions (e.g., Ca²⁺, Mg²⁺) link oppositely charged sites on the soil and the substrate.
Electrostatic adhesion is stronger than simple mechanical adhesion, and therefore such soils are more difficult to remove.
② Chemical Adhesion
Chemical adhesion refers to the bonding of soil to a surface through chemical bonds or hydrogen bonds.
Examples include polar soils, proteins, and rust, which adhere strongly to fibrous materials.
Fibers often contain carboxyl, hydroxyl, and amide groups, which can form hydrogen bonds with the fatty acids and fatty alcohols present in oily soils.
Since chemical bonding forces are relatively strong, soils adhering in this way are firmly attached and hard to remove by ordinary washing.
Special cleaning methods or chemical treatments are often required.
Factors Affecting Soil Adhesion Strength
The degree of adhesion depends on both the nature of the soil and the characteristics of the surface to which it adheres.
Particle size: Smaller solid particles adhere more strongly to surfaces.
Surface type: Particulate soils tend to adhere more easily to fibrous materials.
Polarity: On hydrophilic surfaces (e.g., cotton, glass), polar soils adhere more strongly than nonpolar soils.
Oil-based soils: Nonpolar soils (e.g., oily or greasy substances) generally show stronger adhesion than polar soils such as dust or clay, and are therefore more difficult to remove.
The primary goal of washing is to remove soils and contaminants.
In a medium at a certain temperature (typically water), the physicochemical actions of detergents are used to weaken or eliminate the interactions between soils and the substrate (the soiled material).
Under the influence of mechanical forces—such as hand rubbing, agitation in a washing machine, or water flow—the soil detaches from the surface, achieving the purpose of cleaning.
A. Wetting
Most liquid soils are oily contaminants.
Oil can readily wet and spread over most textile surfaces, forming a thin oil film on the fibers.
The first step of the washing process is therefore the wetting of the surface by the detergent solution.
For simplicity, the fiber surface can be considered a smooth solid surface, where the detergent reduces the surface tension of water, allowing the washing liquid to spread and penetrate more effectively.
B. Detachment and Roll-Up Mechanism
The second step is the removal of the oily film, which occurs through the roll-up mechanism.
Initially, the oil exists as a thin continuous film spread across the solid surface.
As the detergent solution preferentially wets the solid (fiber) surface, the oil film contracts gradually into droplets.
These oil droplets are then displaced by the washing liquid, and under mechanical agitation or shearing forces, they detach completely from the surface and are carried away by the washing medium.
② Mechanism of Removing Solid Soils
Unlike the removal of liquid (oily) soils, which primarily depends on the preferential wetting of the substrate surface by the washing liquid, the removal of solid soils follows a somewhat different mechanism.
During washing, the key process involves the wetting of both the soil particles and the substrate surface by the detergent solution.
Surfactants are adsorbed onto the surfaces of both the solid soil particles and the substrate, thereby reducing the interfacial interaction between them.
This adsorption lowers the adhesion strength of the soil particles on the substrate, making it easier for the soil to detach and be removed.
Role of Electrostatic Effects
Moreover, surfactants—especially ionic surfactants—can significantly influence the surface potential of both the solid soil particles and the substrate.
In aqueous media, most solids and fibrous surfaces carry negative charges, forming diffuse double layers around their surfaces.
Since like charges repel, this electrostatic repulsion weakens the adhesion between soil particles and the surface.
When anionic surfactants are added, they further increase the negative surface potential of both the soil particles and the substrate.
As a result, the repulsive force between them is enhanced, the adhesion strength decreases, and the soils are more easily removed.
Nonionic Surfactants
Nonionic surfactants can also adsorb on charged solid surfaces, though they do not significantly alter the surface potential.
However, the adsorbed nonionic layer can form a protective film of measurable thickness, which helps prevent re-deposition of detached soils onto the cleaned surface.
Cationic Surfactants
In contrast, cationic surfactants tend to reduce or neutralize the negative surface potential of both the soil particles and the substrate.
This diminishes electrostatic repulsion and increases the likelihood of re-adhesion, which is unfavorable for soil removal.
Furthermore, once cationic surfactants are adsorbed, they can render the surface hydrophobic, which reduces wettability and therefore impairs washing efficiency.
③ Removal of Special Soils
Soils such as proteins, starches, human secretions, fruit juice, and tea stains are difficult to remove using ordinary surfactants alone and therefore require special treatment methods.
Protein Soils
Protein-based soils — such as cream, egg, blood, milk, and skin secretions — tend to coagulate and denature upon contact with fibers, forming strongly adherent deposits.
These can be effectively removed using proteolytic enzymes (proteases), which catalyze the hydrolysis of proteins in the soil into water-soluble amino acids or small peptides, allowing them to be washed away.
Starch Soils
Starch soils originate primarily from food sources, such as gravy, pastes, or sauces.
Amylase enzymes can catalyze the hydrolysis of starch into smaller sugar molecules, thereby facilitating their removal during washing.
Fatty Soils
Lipase enzymes are used to catalyze the decomposition of triglyceride-type soils, which are often difficult to remove by conventional methods.
These include sebum, edible oils, and fats.
Lipase breaks triglycerides into glycerol and fatty acids, both of which are more soluble and can be easily removed by detergents.
Colored and Pigmented Stains
Certain colored soils—such as those from fruit juice, tea, ink, or lipstick—are often resistant to complete removal, even after repeated washing.
These stains can be treated using oxidizing or reducing agents (e.g., bleaching powder, hydrogen peroxide, or sodium dithionite), which cause redox reactions that destroy chromophoric and auxochromic groups in the color molecules.
This reaction degrades the pigments into smaller, water-soluble compounds, enabling their removal.
The washing mechanisms discussed above primarily refer to aqueous washing, where water serves as the cleaning medium.
However, due to differences in the types and structures of fabrics, water washing is not always suitable or effective for all garments.
In some cases, water washing may even cause fabric deformation, color fading, or texture changes.
For example:
Most natural fibers absorb water and swell easily, and then shrink during drying, resulting in distortion or deformation.
Woolen fabrics tend to shrink and felt after water washing.
Some wool blends may pill or lose color.
Silk fabrics often become rougher and less lustrous after water washing.
For these reasons, such garments are often cleaned using the dry cleaning method.
Principles of Dry Cleaning
Dry cleaning refers to a washing process that uses organic solvents, particularly nonpolar solvents, instead of water as the medium.
Compared with water washing, dry cleaning is a milder cleaning process.
Because it requires less mechanical agitation, dry cleaning causes minimal damage, wrinkling, or deformation to garments.
Additionally, dry cleaning solvents, unlike water, do not induce fiber swelling or shrinkage, preserving the original shape, color, and texture of the fabric.
When handled properly, dry cleaning can achieve excellent cleaning results while ensuring that garments remain dimensionally stable, colorfast, and long-lasting.
Types of Soils in Dry Cleaning and Their Removal Mechanisms
From the perspective of dry cleaning, soils can generally be classified into three categories based on their solubility characteristics:
① Oil-Soluble Soils
Oil-soluble soils include various oils and greases—either in liquid or semi-solid (greasy) form—which are readily soluble in dry cleaning solvents.
These include animal and vegetable oils, mineral oils, and fats.
The excellent solvency of dry cleaning solvents for oils and greases arises primarily from van der Waals intermolecular forces, which allow efficient dissolution and removal of such soils.
② Water-Soluble Soils
Water-soluble soils can dissolve in water but not in dry cleaning solvents.
They are typically absorbed onto fabrics in aqueous form, and after water evaporation, they precipitate as solid residues such as inorganic salts, starch, and proteins.
To remove these soils during dry cleaning, a small amount of water must be added to the dry cleaning solvent; otherwise, water-soluble contaminants cannot be effectively removed.
However, because water is only slightly miscible with nonpolar dry cleaning solvents, surfactants must be added to improve compatibility.
The presence of water in the dry cleaning solvent enables hydration of both the soil and the fabric surface, facilitating interaction between the polar groups of the surfactant and the surface.
Furthermore, when surfactant micelles form, both water-soluble soils and water can be solubilized within the micelles, allowing them to be carried away by the solvent.
In addition to enhancing the water content, surfactants also prevent redeposition of detached soils, thereby improving the overall cleaning efficiency.
A small amount of water is therefore essential for removing water-soluble soils, but excess water can cause fabric deformation or wrinkling.
Hence, the water content in dry cleaning formulations must be carefully controlled.
③ Non-Soluble Soils (Insoluble in Both Water and Solvent)
Some soils, such as dust, mud, clay, and carbon black, are insoluble in both water and dry cleaning solvents.
These solid particles usually adhere to fabrics by electrostatic attraction or by being bound within oily residues.
In dry cleaning, the mechanical action of the solvent’s flow and turbulence helps dislodge soils held by electrostatic forces.
At the same time, the solvent dissolves the oily components, releasing the solid particles trapped in them.
The small amount of water and surfactant present in the dry cleaning solvent then helps disperse and suspend these detached particles, preventing their redeposition onto the fabric surface.
In summary, effective dry cleaning depends on a balanced interaction among solvent solvency, controlled moisture, and surfactant activity to remove a wide range of soils—whether oil-soluble, water-soluble, or insoluble.
The oriented adsorption of surfactant molecules at interfaces and the reduction of surface or interfacial tension are the primary mechanisms for removing liquid and solid soils.
However, the washing process is quite complex, and even for the same type of detergent, the cleaning performance can be influenced by many additional factors — including detergent concentration, temperature, the nature of the soil, the type of fiber, and the fabric structure.
① Concentration of Surfactants
Micelles formed by surfactants in solution play a crucial role during the washing process.
When the surfactant concentration reaches the critical micelle concentration (CMC), the washing efficiency increases sharply.
Therefore, to achieve good cleaning performance, the detergent concentration should be above the CMC.
However, once the concentration exceeds the CMC, further increases in surfactant concentration result in only minor improvements in washing efficiency.
Excessive addition of surfactant beyond the optimal level is thus unnecessary and uneconomical.
When removing oily soils through solubilization, even above the CMC, the solubilizing capacity of the surfactant continues to increase with concentration.
Hence, it is sometimes beneficial to apply detergent locally on heavily soiled areas (e.g., collars and cuffs), where higher localized concentrations can enhance solubilization and removal of oily contaminants.
② Effect of Temperature
Temperature has a significant influence on the washing process.
In general, increasing temperature promotes soil removal, but excessively high temperatures can sometimes have adverse effects.
Raising the temperature enhances the diffusion of soils, and solid or greasy soils can be more easily emulsified once the temperature exceeds their melting point.
Higher temperatures also cause fiber swelling, which can help detach soils from the fiber surface.
These factors collectively contribute to improved cleaning efficiency.
However, for tightly woven fabrics, excessive fiber swelling can reduce the micro-gaps between fibers, making it more difficult for detergent solution to penetrate and remove soils.
Temperature also affects the solubility of surfactants, their critical micelle concentration (CMC), and the size and number of micelles, thereby influencing the overall washing effect.
For long-chain surfactants, solubility is relatively low at lower temperatures—sometimes even below their CMC value. In such cases, the washing temperature should be appropriately increased.
For ionic surfactants, an increase in temperature typically causes the CMC to rise and the number of micelles to decrease, meaning a higher surfactant concentration is required to maintain cleaning performance.
For nonionic surfactants, increasing temperature tends to decrease the CMC and increase micelle formation, thus enhancing surface activity. However, the temperature should not exceed the cloud point, beyond which surfactant solubility decreases and performance deteriorates.
In summary, the optimal washing temperature depends on both the detergent formulation and the nature of the fabric being cleaned.
Some detergents work effectively at room temperature, while others exhibit significant differences in cleaning power between cold and hot washing conditions.
③ Foam
People often mistakenly associate foaming ability with cleaning performance, assuming that a detergent that produces more foam must clean better.
However, studies have shown that washing efficiency is not directly related to foam volume.
For example, low-foaming detergents can clean just as effectively as high-foaming ones.
Although foam itself does not directly contribute to cleaning, in certain cases, it can aid in soil removal.
For instance:
When washing dishes by hand, the foam in the detergent solution can carry away oil droplets that have been detached from the surface.
When cleaning carpets, foam can trap and lift away dust and solid dirt particles.
Since dust accounts for a large portion of carpet soils, carpet cleaners are generally formulated to have moderate foaming ability.
Foaming ability is also an important factor in shampoos and body washes.
During washing or bathing, fine and creamy foam provides a pleasant, smooth, and comfortable sensation, enhancing the user experience.
④ Fiber Type and Physical Properties of Fabrics
Besides the chemical composition of fibers, the surface morphology, yarn twist, and fabric structure also influence how easily soils adhere to and are removed from textiles.
Wool fibers, with their scaly surface, and cotton fibers, with their flat, twisted ribbon-like shape, tend to trap more soil than smooth fibers.
For example, carbon black adhering to cellulose film (viscose film) can be easily removed, whereas carbon black on cotton fabric is much harder to wash off.
Short-fiber polyester fabrics accumulate more oily soil than long-fiber fabrics, and the oily soils on short-fiber fabrics are also more difficult to remove.
Tightly twisted yarns and densely woven fabrics resist initial soiling because the microscopic gaps between fibers are smaller, making it harder for soils to penetrat.
However, once they do become soiled, these tight structures also hinder the penetration of washing solution, making soil removal more difficult.
Thus, the structure and texture of fabrics can both improve soil resistance and complicate cleaning, depending on how the fibers interact with soil and detergent.
⑤ Water Hardness
The concentration of metal ions such as Ca²⁺ and Mg²⁺ in water has a significant impact on washing performance.
In particular, anionic surfactants tend to form insoluble calcium and magnesium salts when reacting with these ions, which greatly reduces their detergency.
Even when the concentration of surfactant in hard water is relatively high, the cleaning efficiency is still much lower than that in distilled or soft water.
To achieve optimal washing performance, the concentration of Ca²⁺ ions in the water should be below 1 × 10⁻⁶ mol/L (equivalent to less than 0.1 mg/L as CaCO₃).
Therefore, it is necessary to add water softening agents to detergent formulations to remove or sequester Ca²⁺ and Mg²⁺ ions.
NEWSLETTER SIGN UP